This technique emphasizes the significance of a existence cycle approach, which starts with process style and proceeds by process qualification and continued process verification.
In terms of the importance of process validation, it cannot be overstated. It makes certain that a process is able to consistently manufacturing items that meet the desired high-quality and functionality expectations.
By closely monitoring the process, possible challenges might be addressed proactively, reducing the risk of product or service non-conformities and making certain constant merchandise high quality.
It can help recognize and mitigate potential threats and deviations, thereby ensuring the stop goods are Secure, effective, and of the best high quality.
The process qualification phase is significant in setting up confidence within the process's power to continuously develop significant-quality products and solutions. It offers proof which the process is below Manage and capable of meeting the specified outcomes.
Documentation can be a critical factor in the achievement of process validation. SafetyCulture (previously iAuditor) is a knowledge collection and analysis Device made to help it become easier for validation teams to document process-similar facts, execute the validation protocol, and keep all data up-to-date.
Any validation or high quality Skilled Doing the job in everyday life sciences or other click here remarkably regulated industries…
Within this stage, the process is made and documented intimately. The critical process parameters as well as the corresponding functioning ranges are identified.
5. Periodic Validation: Groups consistently Assess the process to examine it really is Operating based on the primary style and design.
Incorporate classes learned and best procedures identified throughout the validation and verification process to more info tell foreseeable future things to do. Make sure compliance with regulatory needs and industry criteria when making ready documentation and reports. Eventually, create a robust technique for Variation Regulate and documentation administration to track revisions and updates correctly.
It's executed only when the producing process hasn't formally been through a documented validation. Retrospective validation is Generally fulfilled with using historic details and trends Investigation to offer proof that the process is at a point out that it is meant for being in.
During the ongoing process verification stage, a variety of process general performance indicators are monitored in order that the process is performing in satisfactory restrictions. These indicators may possibly contain generate, cycle time, process functionality indices, as well as other applicable metrics.
Put together a report analyzing the tendencies in scholar participation in athletics and arts courses over the last 5 years at your school.
Process validation is a systematic solution to make sure that a manufacturing process continuously makes an item of predetermined good quality. During this thorough guideline, We'll investigate the importance of process validation, the key methods involved, regulatory necessities, along with helpful implementation tactics and also the potential issues that could crop up.
 Luke Perry Then & Now!
Luke Perry Then & Now! Mara Wilson Then & Now!
Mara Wilson Then & Now! Tony Danza Then & Now!
Tony Danza Then & Now! Jonathan Lipnicki Then & Now!
Jonathan Lipnicki Then & Now!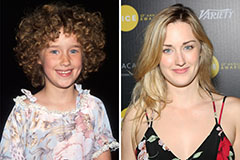 Ashley Johnson Then & Now!
Ashley Johnson Then & Now!